Comparison between eccentric and rotary presses: The right choice for tablet production
Die Herstellung von Tabletten ist ein komplexer Prozess, bei dem die Wahl der richtigen Pressmaschine entscheidend ist. Ob in industriellen...
WORLDWIDE SHIPPING - Order now
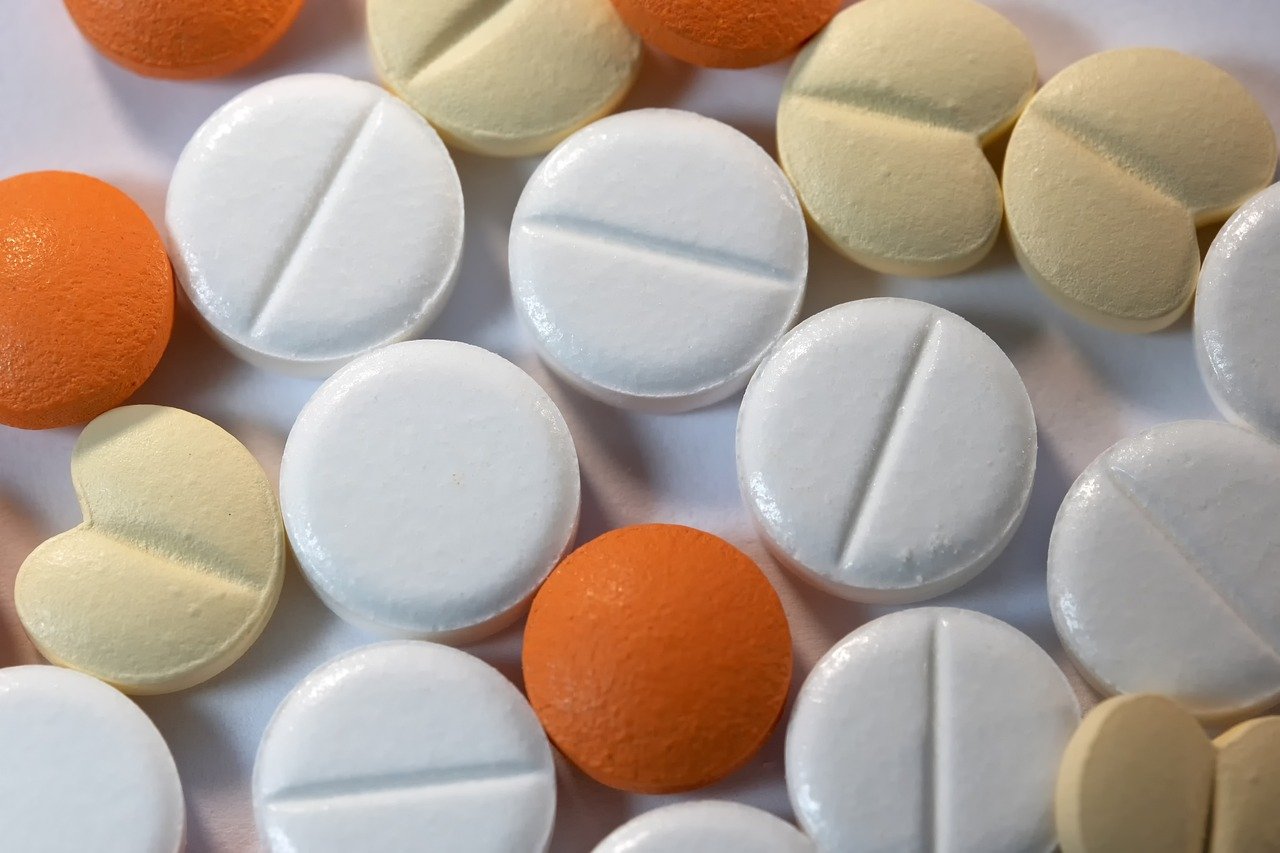
Tablet presses are among the most important machines in the pharmaceutical industry, dietary supplement production, and the chemical and cosmetic sectors. They enable the precise production of tablets of consistent quality, shape, and dosage. However, these machines are complex systems that, despite their high precision, do not operate completely trouble-free. Anyone who operates a tablet press knows that certain problems can occur regularly and often result in time-consuming production interruptions. This makes it all the more important to understand the typical causes and take appropriate measures to correct or prevent them.
One of the most common problems is weight fluctuation. A tablet must be produced within very tight tolerances, as consistent dosage of the active ingredient is crucial for the quality and safety of the product. However, deviations often occur due to uneven filling of the die. This can be caused by insufficient powder flowability, moisture, or incorrect adjustment of the filling system. Wear and tear on the dosing mechanisms also frequently leads to tablets that are either too heavy or too light. Regular machine calibration, optimization of powder flow properties, and consistent maintenance contribute significantly to solving this problem.
Another well-known problem is capping. This occurs when the upper or lower tablet surface chipped or detaches. This phenomenon is particularly common at high compression pressures. The causes are usually insufficient deaeration of the powder or excessive moisture trapped during the compression process. Uneven pressure buildup within the die can also be responsible. To reduce the risk of capping, a detailed analysis of the powder mixture is necessary. It also helps to precisely adjust compression parameters such as pre-compaction or main pressure and to adapt the formulation if necessary.
No less widespread is the problem of sticking, the adhesion of powder particles to the press's punches. This leads to irregular surfaces, impairs tablet quality, and can damage the machine. Sticking is particularly critical when it is caused by sticky or hygroscopic components in the mixture. Inadequate lubrication or excessively high ambient temperatures further exacerbate this effect. Special non-stick coatings on the punches, adjustment of the lubricants in the formulation, and careful control of the climatic conditions in the production environment can help.
A fourth common problem is insufficient tablet hardness. While tablets that are too hard can negatively impact dissolution time and limit bioavailability, tablets that are too soft are mechanically unstable and tend to break during packaging or transport. Hardness is influenced by numerous factors: the composition of the mixture, the pressing force, the residence time of the powder under pressure, and the moisture content. To achieve an optimal balance, it is necessary to analyze the powder characteristics, carefully select binders, and precisely adjust the pressing parameters. Continuous quality control during production ensures that deviations are detected and corrected in a timely manner.
The fifth key problem concerns the uneven thickness or defective appearance of the tablets. Visual deviations are particularly common in multi-layer tablets or with special shapes. Causes include uneven powder flow, wear on the dies or punches, and incorrect synchronization between the pre-compression and main compression stages. Since visual quality is crucial for end-user confidence, these sources of error must be identified quickly. Regular tool inspections, the replacement of worn components, and monitoring the filling speed contribute significantly to maintaining stable tablet quality.
While the problems mentioned are typical, they can be managed through a combination of preventative maintenance, optimized powder preparation, and precise machine operation. Those who regularly clean their tablet presses, replace wear parts in a timely manner, and consistently monitor production parameters can significantly reduce downtime while ensuring consistently high product quality. Investing in modern measurement and diagnostic technology that detects deviations at an early stage is also worthwhile. Ultimately, experience shows that not only the machine itself but also the qualifications of the operating personnel are crucial: Trained employees are able to identify problems early and respond appropriately. This transforms a potential weak point into a stable and efficient production line that meets both regulatory requirements and customer expectations.
FAQ
Die Herstellung von Tabletten ist ein komplexer Prozess, bei dem die Wahl der richtigen Pressmaschine entscheidend ist. Ob in industriellen...
Der Kauf einer Tablettenpresse ist eine strategische Entscheidung für Unternehmen aus der Pharma-, Chemie-, Lebensmittel- oder Nahrungsergänzungsmittelindustrie. Die richtige Maschine...
Der Einsatz einer Tablettenpresse erfordert nicht nur technisches Verständnis, sondern auch sorgfältige Vorbereitung und Aufmerksamkeit. Obwohl diese Maschinen für die...
Die Herstellung von Tabletten ist ein komplexer Prozess, bei dem jede Komponente des Tablettenpressens entscheidend für die Qualität des Endprodukts...
